Abstract
Background: Vaso-occlusive crises (VOCs) are the hallmark of SCD. VOCs and silent vaso-occlusion can lead to complications (eg acute chest syndrome, hepatic/renal dysfunction, chronic pain, multi-organ failure) and premature death. Crizanlizumab, an anti-P-selectin monoclonal antibody (mAb), is authorized in >40 countries to prevent/reduce VOCs in SCD pts aged ≥16 yrs.
IRRs are defined as any signs/symptoms (S/S) experienced by pts during/within 24 hrs of infusion of a pharmacologic/biologic agent. IRRs are quite common with mAbs (frequency 1.6‒99%; Rombouts et al Anticancer Res 2020). S/S of IRRs vary; pain events, such as headache, back pain, myalgia, chest pain and joint pain, have been described as S/S of IRRs. Although pain events are known adverse drug reactions in the crizanlizumab label (eg arthralgia, myalgia, pain at various locations), due to data limitations and confounding manifestations of SCD, pain events occurring during/within 24 hrs of crizanlizumab infusion in SUSTAIN were not identified as potential IRRs (Ataga et al N Engl J Med 2017). For pts receiving crizanlizumab, IRR-related pain events may differ in location, severity and/or nature from a pt's usual SCD/VOC pain.
Aim: To review data on IRRs presenting as pain events in SCD pts treated with crizanlizumab via reports received by Novartis since approval in Nov 2019.
Methods: Data sources included PM reports from providers (spontaneous) and reports from the managed access/pt orientation program. To obtain the reports (which could include ≥1 event), a cumulative custom search of the Novartis safety database was performed up to Jun 2021, using ~111 MedDRA terms associated with potential S/S of IRRs presenting as pain events. IRRs must have occurred during/within 24 hrs of the most recent crizanlizumab infusion, and pain could differ from a pt's usual SCD/VOC, with/without other S/S. IRR incidence was measured by the reporting rate (RR). Reports were not gathered via a uniform data collection system, so there are limitations, including potential underreporting, incompletely documented cases, or bias towards reporting severe events.
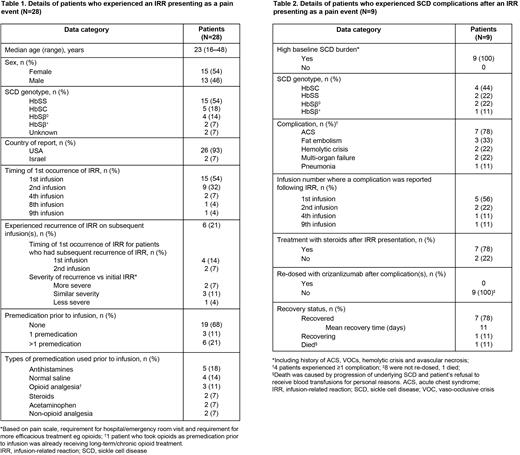
Results: IRRs presenting as pain events were experienced by 28 pts (Table 1); the most common S/S were back pain, pain in extremity, arthralgia, musculoskeletal chest pain and headache. RR was 1.67 cases per 100 pt-yrs (95% CI 1.11‒2.42). Most pts (n=24) initially experienced IRR at the 1st or 2nd infusion, and the majority recovered within 3 days. IRR recurred on subsequent infusion(s) in 6 pts.
Of the 28 pts, 20 (71%) were hospitalized for further treatment, including analgesics, antihistamines, IV fluids and/or steroids. Nine pts (32%) reported SCD complications after IRR (Table 2). Crizanlizumab was discontinued in 23 pts (82%) after their most recent IRR occurrence, including all pts who experienced secondary SCD complications.
Discussion: Comprehensive investigation identified 28 pts with reported IRRs presenting as pain events that had a potential causal relationship with crizanlizumab infusion based on temporality. All pts recovered or are recovering, except 1 who had SCD complications and refused blood transfusions for personal reasons. Most pts had initial IRR at the 1st or 2nd infusion and discontinued crizanlizumab after initial IRR; 6 experienced recurrent IRRs on subsequent infusion(s). Resolution time was prolonged for pts who reported known SCD complications following IRR. Causal association of complications following IRR was confounded by the underlying disease and use of steroids to treat IRRs. Systemic corticosteroid exposure in SCD pts has been associated with pain and other complications, from severe VOCs to hemorrhagic stroke and death. No data are available regarding whether the 28 pts had an active VOC or other SCD complications prior to receiving crizanlizumab.
Conclusions: Although rare, based on review of PM data, healthcare professionals should be aware of the possibility of IRRs presenting as pain events during or after any crizanlizumab infusion. Crizanlizumab labels have been/are being updated by Novartis to provide information on monitoring for S/S of IRRs presenting as pain events, and guidance on management/prevention of subsequent IRRs, including a statement recommending caution when using corticosteroids in SCD pts. Given the limited data regarding IRRs and predictability of complications, Novartis is committed to further understanding these events.
Kanter: Fulcrum Therapeutics, Inc.: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Forma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beam: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Graphite Bio: Consultancy; GuidePoint Global: Honoraria; Fulcrum Tx: Consultancy. Shah: Johnson & Johnson: Current equity holder in publicly-traded company; Novartis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Joshi: Novartis Healthcare Private Limited: Current Employment. Mehta: Novartis Healthcare Pvt. Ltd.: Current Employment. Levine: Biontech: Current equity holder in publicly-traded company; Novartis: Current Employment, Current equity holder in publicly-traded company. Arunagiri: Novartis Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Paulose: Novartis Pharmaceuticals Corporation: Current Employment. Donohue: Novartis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Scalera: Novartis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Manwani: Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal